Taken with each other, lyophilization is often a precious tool with the preservation of Organic samples with many rewards. We purpose to attract interest into the big selection of choices supplied by freeze drying in pre-clinical or fundamental exploration.
Manufacturing methods and continual top quality improvement that established the typical inside the pharma industry.
The item’s formulation should be thoroughly intended to ensure that it truly is appropriate for lyophilization since the composition in the item, which includes buffers, excipients, and the selection of cryoprotectants, will considerably affect cycle enhancement.
Licence this book for your library Study institutional subscriptions Other approaches to entry
Pulverization of dried samples may be attained which has a ceramic mortar as well as a pestle as well, but a TissueLyser device with metal beads will also be utilised. Metallic beads is usually conveniently eliminated that has a magnet minimizing sample reduction.
test for all parametrical comparisons, or in the case of nonparametric data by Wilcoxon check on ranks. To test homogenization performance, variances in just each group have been in comparison by Levene’s exam done in Microsoft Business Excel (Microsoft, Redmond, WA, United states) in which one factor ANOVA was placed on complete variances of values to mean. Significance was set a priori at P
We're uniquely positioned to create lyophilization cycles from the start or to improve present cycles, offering a commercially fascinating nonetheless inexpensive process. Having designed more than 675 lyophilization cycles, shopper partners depend on us to attain their Excellent Focus on Merchandise Profile and produce everyday living-switching therapies to individuals.
Firstly from the lyophilization process, products should be formulated in this kind of way that they are acceptable to undertake thermal remedy. This usually consists of the inclusion of cryoprotectants including saccharides and polyols to guard the item all through freezing.
cryopreservation of pharmaceuticals freeze drying pharmaceuticals QbD scale up pharmaceutical processes biopharmaceuticals biologics drying systems pharmaceutical sciences antibody drug conjugates CART (chimeric antigen receptor modified T-Mobile) BITES (Bispecific T Mobile ) Search inside of this reserve
Lyophilization is really a process that consists of freezing a liquid drug item and after that taking away the frozen solvent by means of sublimation, more info delivering a secure good matrix of drug solution as well as other excipients.
As an inaugural member from the Process Advancement group, Matt now manages very expert scientists in the same group, leveraging his process information and specialized prowess to inform experts and clientele alike, from smaller scale preclinical assessments to late-phase characterization and aseptic fill-complete. Matt acquired his B.S. in Chemical Engineering in the College of Massachusetts.
e. the temperatures during which the product undergoes a thermodynamic adjust in condition via glass transition, recrystallization, and eutectic soften. Even a qualitative change of point out observed by means of FDM (collapse onset) is essential to the characterization of the products. The moment recognized, the main focus is positioned back to the lyophilization cycle parameters, and temperature and vacuum ranges are suggested to ensure solution high-quality and prevent failure.
As soon as Main drying is effectively entire, the process has normally eradicated in between ninety-95% of your solvent and developed a physically steady lyophilized matrix. You can find a person dilemma, nonetheless; There's normally remaining solvent that is certainly sure among crystals that can't be totally removed from the Electrical power input of sublimation by yourself. The ultimate section – secondary drying, entails further elimination in the residual humidity while in the lyophilized item by expanding the temperature and eliminating bound solvent via desorption.
As we've journeyed in the nuances of the technological innovation, lyophilization pharmaceutical products we've seen how it's vital to get a plethora of applications—from preserving the potency of pharmaceuticals to the prolonged shelf lifetime of foodstuff.
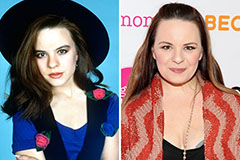 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Earvin Johnson III Then & Now!
Earvin Johnson III Then & Now! Raquel Welch Then & Now!
Raquel Welch Then & Now! Bo Derek Then & Now!
Bo Derek Then & Now! Rachael Leigh Cook Then & Now!
Rachael Leigh Cook Then & Now!